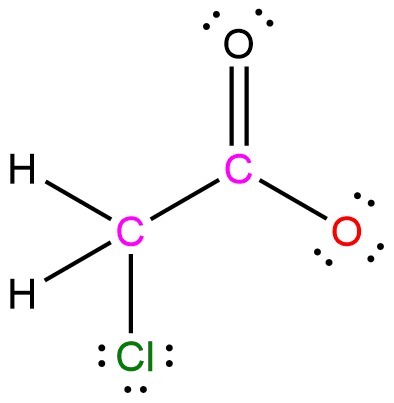
For example, what is the electronegativity difference for Acetone(CH2O)? Are there two different answers? 0.4 for C & H, and 1.0 for C & O? Which one do you choose?
6 Comments
AlwaysReady1
•
Apr 3, 2016, 10:14 PM
I might not understand very well the question but if you are trying to find an electronegativity for a compound to try to assess if it would attract electrons I think there are other factors that would affect this.
I would say that depending on the compound. In the case of CH2O, which would be formaldehyde, you could see that the oxygen has two pairs of electrons available to be donated. Neither H or C would be available for bonding since they have all the bonds necessary to fill the valence shell.
Robo94
•
Apr 4, 2016, 10:22 AM
You're trying to apply something from a binary system to a bigger system. I assume you're trying to find the dipole moment of a molecule. In a biatomic molecule, (A bonded to B) you can just say the potential difference is that of A minus that of B. Bigger molecules require a lot more math per atom.
If you're asking because you need homework help its a completely different process than what you're used to. I suggest starting by figure out how to do it with Water, and working your way out from there.
Watch this: https://www.khanacademy.org/science/organic-chemistry/gen-chem-review/electronegativity-polarity/v/dipole-moment
Philosoaxolotl
•
Electronegativity is a concept that is designed for individual elements (individual atoms really), and isn't really applicable to molecules.
What exactly are you trying to use this information for? If you're looking at how electrons will transfer between molecules, there's a little bit more going on - within a molecule, more electronegative elements can pull electrons away from other atoms (this is common in organic molecules, for example, where oxygen often bonds to carbon and will pull some of its electrons away). However, this effect is reduced in longer molecules. It's a more complex system because molecules don't have one constant electronegativity (which you can approximately say is true for atoms), but instead have more localized regions of charge at different locations on the molecule that will react differently.
It sounds to me like your question is about the electronegativity difference between atoms of an acetone molecule. For this, it definitely depends on the two atoms you're looking at, and will not be constant throughout - however, it will also notsimply be the difference you'd calculate from an electronegativity table because of the effects mentioned above.
This was kind of a vague explanation and I'm only an undergrad so take my words with a grain of salt, but feel free to ask me to elaborate.
cheeseborito
•
This is wrong.
Electronegativity is by definition the pull an atom has on the electrons in a covalent bond with another atom. So, in reality, an element does not have one standard electronegativity, and its measured electronegativity will vary based on what it is bound to. We can't talk about the electronegativity of one atom in a vacuum.
That isn't to say we can't speak in averages, and for all intents and purposes (Though not technically), the effective electronegativity of an oxygen atom bound to a carbon atom will be more or less the same.
As far as I'm aware, while my definition of electronegativity may not be flawless, the pull of an oxygen atom on the electrons of a carbon atom is not independent of what the carbon is bound to. The effective local charge around the oxygen in acetic acid, for example, would be higher than that of the oxygen in decanoic acid.
The electronegativity thing may have been poor phrasing on my part - I didn't mean individual atoms in a vacuum, but rather individual pairs of atoms relative to one another. An oxygen will always exert the same pull relative to a carbon but the relative difference in local charge will vary because of other atoms exerting a pull - thus the things we typically use electronegativity to understand become more complicated.
6 Comments
AlwaysReady1
•
Apr 3, 2016, 10:14 PM
I might not understand very well the question but if you are trying to find an electronegativity for a compound to try to assess if it would attract electrons I think there are other factors that would affect this.
I would say that depending on the compound. In the case of CH2O, which would be formaldehyde, you could see that the oxygen has two pairs of electrons available to be donated. Neither H or C would be available for bonding since they have all the bonds necessary to fill the valence shell.
Robo94
•
Apr 4, 2016, 10:22 AM
You're trying to apply something from a binary system to a bigger system. I assume you're trying to find the dipole moment of a molecule. In a biatomic molecule, (A bonded to B) you can just say the potential difference is that of A minus that of B. Bigger molecules require a lot more math per atom.
If you're asking because you need homework help its a completely different process than what you're used to. I suggest starting by figure out how to do it with Water, and working your way out from there.
Watch this: https://www.khanacademy.org/science/organic-chemistry/gen-chem-review/electronegativity-polarity/v/dipole-moment
Philosoaxolotl
•
Electronegativity is a concept that is designed for individual elements (individual atoms really), and isn't really applicable to molecules.
What exactly are you trying to use this information for? If you're looking at how electrons will transfer between molecules, there's a little bit more going on - within a molecule, more electronegative elements can pull electrons away from other atoms (this is common in organic molecules, for example, where oxygen often bonds to carbon and will pull some of its electrons away). However, this effect is reduced in longer molecules. It's a more complex system because molecules don't have one constant electronegativity (which you can approximately say is true for atoms), but instead have more localized regions of charge at different locations on the molecule that will react differently.
It sounds to me like your question is about the electronegativity difference between atoms of an acetone molecule. For this, it definitely depends on the two atoms you're looking at, and will not be constant throughout - however, it will also notsimply be the difference you'd calculate from an electronegativity table because of the effects mentioned above.
This was kind of a vague explanation and I'm only an undergrad so take my words with a grain of salt, but feel free to ask me to elaborate.
cheeseborito
•
This is wrong.
Electronegativity is by definition the pull an atom has on the electrons in a covalent bond with another atom. So, in reality, an element does not have one standard electronegativity, and its measured electronegativity will vary based on what it is bound to. We can't talk about the electronegativity of one atom in a vacuum.
That isn't to say we can't speak in averages, and for all intents and purposes (Though not technically), the effective electronegativity of an oxygen atom bound to a carbon atom will be more or less the same.
As far as I'm aware, while my definition of electronegativity may not be flawless, the pull of an oxygen atom on the electrons of a carbon atom is not independent of what the carbon is bound to. The effective local charge around the oxygen in acetic acid, for example, would be higher than that of the oxygen in decanoic acid.
The electronegativity thing may have been poor phrasing on my part - I didn't mean individual atoms in a vacuum, but rather individual pairs of atoms relative to one another. An oxygen will always exert the same pull relative to a carbon but the relative difference in local charge will vary because of other atoms exerting a pull - thus the things we typically use electronegativity to understand become more complicated.
6
0